
Perivascular Research Group
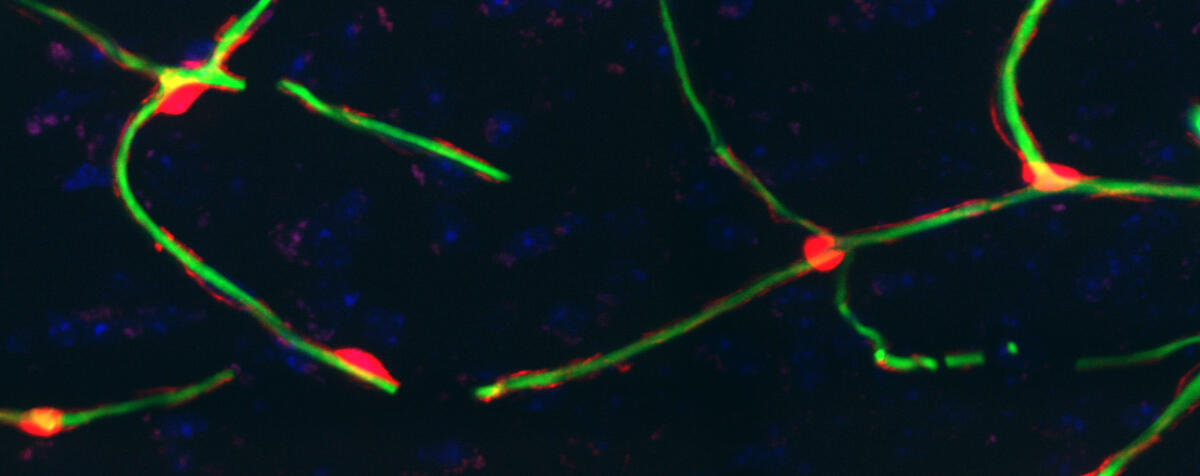
Our group aims to understand how the vascular system contributes to the function of the brain and other organs, and how this becomes dysfunctional in disease.We perform laboratory-based research investigating the contribution of the vascular system to the health of the brain and other organs including skeletal muscle and the placenta. For tissue cells (e.g. neurons) to function, they require energy which is supplied by the vascular system but the mechanisms that control this locally are complex. We currently focus our research on a cell on capillaries called pericytes, which can regulate capillary blood flow and therefore organ function. We are interested in how pericytes function, communicate between the tissue and the blood vessel, signalling pathways controlling this, and how these processes go wrong in diseases such as stroke and Alzheimer’s disease.The Perivascular Research Group is led by Associate Professor Brad Sutherland at the University of Tasmania.
Some of our current projects:
Blood vessels squeezed to death by pericytes: a new therapeutic target for ischaemic stroke.
This project will use in vitro and in vivo models to assess how pericytes, a specific cell that controls blood flow in the brain, are affected by stroke and will identify mechanisms that could be targeted as a treatment strategy for stroke.Pericyte dysfunction limiting energy supply in Alzheimer’s disease.
One possible cause of Alzheimers disease (AD) is narrowing of small blood vessels (capillaries) within the brain, limiting blood flow and energy supply. Pericytes, a cell found only on capillaries, maintain blood flow throughout the brain. In AD, pericytes may die leading to an energy deficit and memory problems. We are using human brains and animal models to determine whether pericyte loss causes AD and how this is happening.The role of pericytes in placental function and perinatal outcome. This project will assess how pericytes, a specific cell that may control blood flow in placenta, could contribute to restricted growth of babies during pregnancy.
Our Team

Associate Professor Brad Sutherland
Brad is an Associate Professor of Neuroscience and leads the Perivascular Research Group (PVRG) in the Tasmanian School of Medicine, University of Tasmania, Tasmania, Australia. Brad completed his PhD at the University of Otago (Dunedin, New Zealand) where he investigated the activation of inflammatory pathways in the brain after stroke. He then took up a Post-doctoral Research Fellowship with the Acute Stroke Programme, Radcliffe Department of Medicine, University of Oxford (Oxford, UK). During his time in Oxford, he formed an interest in the regulation of blood flow in the brain in health and disease. This led to studies investigating the interaction between brain tissue and the blood vessels, the signalling mechanisms that controlled energy delivery to the brain, and how these were disrupted in conditions such as stroke. In mid-2016, Brad was recruited to the University of Tasmania to build a neurovascular research program to continue his research into cerebral blood flow and brain diseases such as stroke and dementia. He uses a wide range of in vitro and in vivo models to assess mechanisms of neurovascular regulation and models of disease.

Dr Gary Morris
Gary is a Research Fellow working in the Perivascular Research Group led by Assoc. Prof. Brad Sutherland at the University of Tasmania. He completed an MSc on the biochemistry of Alzheimer’s disease at the University of Otago, NZ, before moving to Sydney where he completed a PhD on stroke and Alzheimer’s disease at the University of New South Wales. Gary’s research focuses on how blood vessels and pericytes function in the healthy brain and how their injury contributes to stroke and Alzheimer’s disease. He is particularly interested in the interaction between the immune cells of the brain (microglia) and pericytes.

Dr Jo-Maree Courtney
Jo-Maree is a researcher and lecturer at the Tasmanian School of Medicine. She completed a PhD in developmental biology at Kings College London. Jo-Maree's research interests include the interactions between pericytes and other cells of the neurovascular unit and the role of pericytes in female reproductive system.

Dr Lachlan Brown
Lachlan is a Junior Research Fellow in the Perivascular Research Group (PVRG) led by Assoc. Prof. Brad Sutherland at the University of Tasmania. He completed an Honours degree investigating mechanisms of axon pathfinding in response to novel guidance cues at the University of Tasmania, before joining Brad, where he completed a PhD on stroke and neurovascular signalling and was a key member in establishing many of the techniques used by the PVRG lab. Lachlan’s current research focuses on understanding how pericyte contractility and microvascular blood flow changes can influence neurovascular signalling. In particular, he is interested in exploring these changes in models of stroke, chronic fatigue, and Alzheimer’s disease.

Dr Natalie King
Natalie recently completed her PhD in the Perivascular Research Group. She is researching the role of neurovascular dysfunction in neurodegenerative diseases including Alzheimer’s disease and Multiple Sclerosis. Natalie’s current research focuses on the use of stem cells to study the role of the genetic risk factor APOE ε4 in vascular dysfunction in Alzheimer’s disease with a particular focus on pericyte changes. She is particularly interested in applying cell culture techniques to understand the molecular basis of disease.

Dr Jake Cashion
Jake recently completed his PhD in the Perivascular Research Group. He is interested in the role of pericytes within the neurovascular unit, and how pericyte dysfunction may contribute to neurodegenerative diseases such as multiple sclerosis, Alzheimer’s disease, and stroke. Jake completed his undergraduate in biochemistry and microbiology at the University of Tasmania in 2018, where he began working with the PVRG. He later went on to complete his honours with the PVRG in 2019 exploring the impact of chronic cerebral hypoperfusion on pericyte function in Alzheimer’s disease using both in vitro and in vivo models.More recently, through his PhD, Jake is investigating whether pericyte loss can contribute to pathology seen in multiple sclerosis such as demyelination, oligodendrocyte progenitor cell dysfunction, leukocyte infiltration and the loss of glial-vascular relationships. Jake works closely with the Glial Research Team at the Menzies Institute for Medical Research, led by Prof. Kaylene Young.

Hannah Coombe
Hannah Coombe is a PhD candidate in the Perivascular Research Group (PVRG). She is interested in the role of pericytes in the function of the neurovascular unit and how this is affected by ischaemic stroke and ageing. She is using in vivo models of stroke and various transgenic approaches to better understand the cellular and vascular responses to ischaemic stroke. Prior to her PhD, Hannah completed her undergraduate degree in Medical Research in 2019 at the University of Tasmania majoring in neuroscience. She then undertook an honours year with the PVRG investigating the roles of pericytes in fetal growth restriction in human placentas, where she achieved first class honours. Hannah aims to finish her PhD in 2024.
Recent Publications
2023Brown, L. S., King, N. E., Courtney, J. M., Gasperini, R. J., Foa, L., Howells, D. W., Sutherland, B. A.
Brain pericytes in culture display diverse morphological and functional phenotypes,
Cell Biol Toxicol.
Full TextMorris, Gary P., Catherine G. Foster, Jo-Maree Courtney, Jessica M. Collins, Jake M. Cashion, Lachlan S. Brown, David W. Howells, Gabriele C. DeLuca, Alison J. Canty, Anna E. King, Jenna M. Ziebell, and Brad A. Sutherland.
Microglia directly associate with pericytes in the central nervous system
Glia 71:1847-1869
Full TextMorris, Gary P., Emma K. Gowing, Jo-Maree Courtney, Hannah E. Coombe, Natalie E. King, Sarah S.J. Rewell, David W. Howells, Andrew N. Clarkson, Brad A. Sutherland
Vascular perfusion differs in two distinct PDGFRβ-positive zones within the ischemic core of male mice 2 weeks following photothrombotic stroke,
Journal of Neuroscience Research, 101:278-292
Full TextMorris, Gary P. and Sutherland, Brad A
The presence of functional blood vessels in the ischemic core provides a therapeutic target for stroke recovery
Neural Regeneration Research 18:2653-2654
Full TextPremilovac D and Sutherland BA
Acute and long-term changes in blood flow after ischemic stroke: challenges and opportunities
Neural Regeneration Research 18:799-800
Full TextCashion JM, Young K, and Sutherland BA
How does neurovascular unit dysfunction contribute to multiple sclerosis?
Neurobiology of Disease 178:1-17
Full Text2022Courtney, J. M., G. P. Morris, E. M. Cleary, D. W. Howells, and B. A. Sutherland
Automated Quantification of Multiple Cell Types in Fluorescently Labeled Whole Mouse Brain Sections Using QuPath
Bio Protocol, 12.
Full TextKing, Natalie E., Jo-Maree Courtney, Lachlan S. Brown, Catherine G. Foster, Jake M. Cashion, Emily Attrill, Dino Premilovac, David W. Howells, and Brad A. Sutherland
Pharmacological PDGFRβ inhibitors imatinib and sunitinib cause human brain pericyte death in vitro
Toxicology and Applied Pharmacology, 444: 116025.
Full TextRussell, Ash Allanna Mark, Brad A Sutherland, Lila M Landowski, Malcolm Macleod, and David W Howells.
What has preclinical systematic review ever done for us?
BMJ Open Science, 6: e100219.
Full Text2021Courtney, J. M., G. P. Morris, E. M. Cleary, D. W. Howells, and B. A. Sutherland
An automated approach to improve the quantification of pericytes and microglia in whole mouse brain sections
eNeuro, 8.
Full TextFoster, C. G., L. M. Landowski, B. A. Sutherland, and D. W. Howells
Differences in fatigue-like behavior in the lipopolysaccharide and poly I:C inflammatory animal models
Physiol Behav, 232: 113347.
Full TextSutherland, Brad A
The Complex and Integral Roles of Pericytes Within the Neurovascular Unit in Health and Disease
in Alexander Birbrair (ed.), Biology of Pericytes – Recent Advances (Springer International Publishing: Cham).
Full Text2020Attrill, E., C. Ramsay, R. Ross, S. Richards, B. A. Sutherland, M. A. Keske, E. Eringa, and D. Premilovac.
Metabolic-vascular coupling in skeletal muscle: A potential role for capillary pericytes?
Clin Exp Pharmacol Physiol, 47: 520-28.
Full TextBeard, Daniel J, Lachlan S Brown, and Brad A Sutherland
The rise of pericytes in neurovascular research
Journal of Cerebral Blood Flow & Metabolism, 40: 2366-73.
Full TextCourtney, J. M., and B. A. Sutherland
Harnessing the stem cell properties of pericytes to repair the brain
Neural Regen Res, 15: 1021-22.
Full TextLandowski, L. M., B. Niego, B. A. Sutherland, C. E. Hagemeyer, and D. W. Howells
Applications of Nanotechnology in the Diagnosis and Therapy of Stroke
Semin Thromb Hemost, 46: 592-605.
Full TextPremilovac, D., S. J. Blackwood, C. J. Ramsay, M. A. Keske, D. W. Howells, and B. A. Sutherland
Transcranial contrast-enhanced ultrasound in the rat brain reveals substantial hyperperfusion acutely post-stroke
J Cereb Blood Flow Metab, 40: 939-53.
Full Text2019Brown, Lachlan S., Catherine G. Foster, Jo-Maree Courtney, Natalie E. King, David W. Howells, and Brad A. Sutherland
Pericytes and Neurovascular Function in the Healthy and Diseased Brain
Front Cell Neurosci, 13: 282.
Full Text